metallic bonds form between
The electrons within delocalise and are not attached to any particular metal atom. Because of the overlapping metallic.
:max_bytes(150000):strip_icc()/plasma-ball-133939598-5c49e2c746e0fb0001472483.jpg) |
| Definition And Properties Of Metallic Bonding |
Their outermost energy levels overlap.
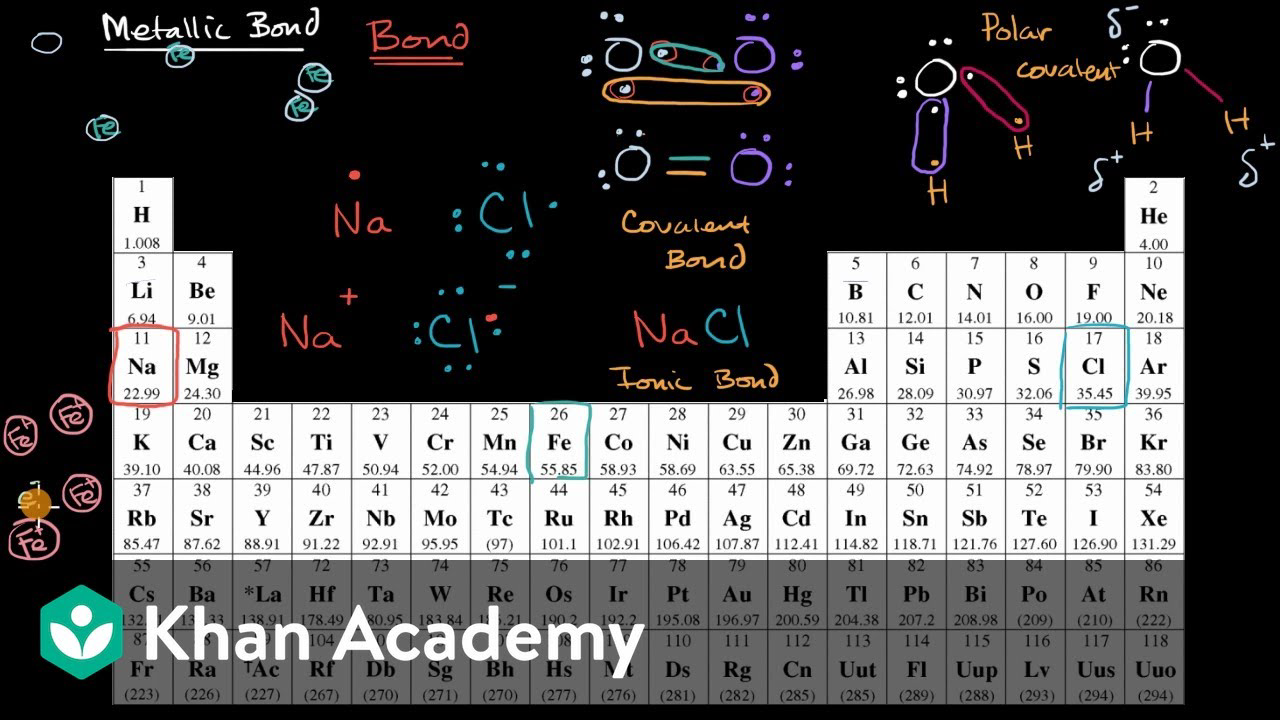
. Covalent bonds occur between two non-metals metallic bonds is between two metals while ionic is observed between non-metal and metal. Graphene is an example of two-dimensional metallic bonding. Which statement correctly describes metallic bonds. Mostly in the periodic table left elements form metallic bonds.
Metallic bonds are formed when atoms share electrons in a way that creates a sea of delocalized electrons. Covalent bonds involve sharing. Its metallic bonds are similar to aromatic bonding in benzene naphthalene anthracene ovalene etc. Metallic bonds are a variation on covalent bonding where the outer orbitals of the metal atoms all overlap allowing the.
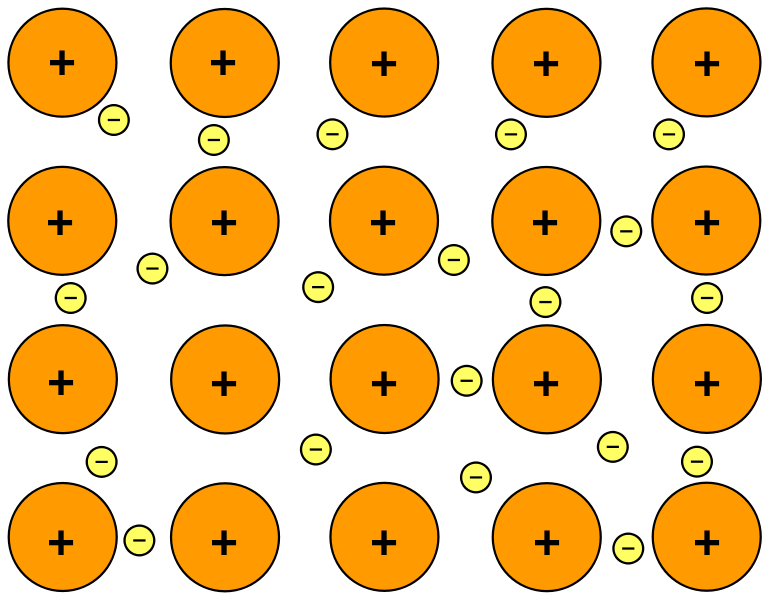
They involve an attraction between. The combination of two phenomena gives rise to metallic bonding. This forms positive metal ions within a sea. Metallic bonds are formed only between metal atoms.
Metals such as silver iron gold aluminium are bonded by metallic bonds via delocalised electrons. They form when certain atoms lose electrons and other atoms gain electrons. The latter could be called electron deficiency. A metallic bond is an impact that holds the metal ions together in the metallic object.
This bond occurs between metallic elements. Ionic bonds are typically stronger than covalent bonds but weaker. It is a force of attraction between the metallic cations and the delocalised electrons. What is a Metallic Bond.
Metallic bonding is the type of chemical bonding that occurs between atoms of metals. Ionic bonds connect metals to nonmetals and metallic bonds connect a large number of metal atoms. As far as I understand metals form metallic bonds because they cannot share electrons therefore they almost fuse their outer shells to form giant seas of delocalised electrons. Delocalization of electrons and the availability of a far larger number of delocalized energy states than of delocalized electrons.
In a metallic bond atoms share their electrons in a way that allows them to form a. Model Showing Metallic Bonding The bonding in metals is a result of the closeness of many metal atoms. Metals form bonds by merging their outer shell electron orbitals. Metallic bonds occur when there are delocalized electrons that move freely throughout a metal lattice.
Metallic bonds are formed when the charge is spread over a larger distance as compared to the size of single atoms in solids. Metallic bonds form between dissimilar metals in alloys. The delocalized electrons allow the atoms to move. Furthermore we also learned that these bonds are non-directional.
Metallic bonds are formed by valence electrons that interchange freely throughout the metallic crystalline structure.
 |
| Difference Between Ionic Bonding And Metallic Bonding Compare The Difference Between Similar Terms |
 |
| Chemical Bonding Comic Strip Storyboard By 63b9e56b |
 |
| Bonding Intro Reading Notes Bonding The Two Main Types Of Bonds Formed Between Atoms Are Ionic Studocu |
 |
| Metallic Bonding Gcse Chemistry Combined Science Aqa Revision Study Rocket |
 |
| Metallic Bonds Properties Examples Explanation Of Metallic Bonds |
Posting Komentar untuk "metallic bonds form between"